A vacuum is a volume of space that is empty of matter, including air, so that gaseous pressure is much less than standard atmospheric pressure. The root of the word vacuum is the Latin word vacuus (pl. vacua) which means "empty," but space can never be perfectly empty.
Explained[]
A perfect vacuum with a gaseous pressure of absolute zero is a philosophical concept with no physical reality; see sections below on Vacuum in Space. Wikipedia:Physicists often discuss ideal test results that would occur in a perfect vacuum and use the term partial vacuum to refer to real vacuum. In engineering it refers to partial vacuum only.
Vacuum Quality[]
The quality of a vacuum is indicated by the amount of matter remaining in the system. For industrial purposes, vacuum is primarily measured by its absolute pressure, but a complete characterization requires further parameters, such as temperature and chemical composition. One of the most important parameters is the mean free path (MFP) of residual gases, which indicates the average distance that molecules will travel between collisions with each other. As the gas density decreases, the MFP increases, and when the MFP is longer than the chamber, pump, spacecraft, or other objects present, the continuum assumptions of fluid mechanics do not apply. This vacuum state is called high vacuum, and the study of fluid flows in this regime is called particle gas dynamics. The MFP of air at atmospheric pressure is very short, 7×10−8m, but at 100 mPascal (~1×10−3 Torr) the MFP of room temperature air is roughly 10 cm, which is on the order of everyday objects such as vacuum tubes. The Crookes radiometer turns when the MFP is larger than the size of the vanes.
Vacuum quality is also subdivided into ranges according to the technology required to achieve it or measure it. These ranges do not have universally agreed definitions (hence the gaps below), but a typical distribution is as follows:
| Atmospheric pressure | 760 Torr | 101 kPa |
| Low vacuum | 760 to 25 Torr | 100 to 3 kPa |
| Medium vacuum | 25 to 1×10−3 Torr | 3 kPa to 100 mPa |
| High vacuum | 1×10−3 to 1×10−8 Torr | 100 mPa to 1 µPa |
| Ultra high vacuum | 1×10−9 to 1×10−12 Torr | 100 nPa to 100 pPa |
| Extremely high vacuum | <1×10−12 Torr | <100 pPa |
| Outer Space | 1×10−6 to <3×10−17 Torr | 100 nPa to <3fPa |
| Perfect vacuum | 0 Torr | 0 Pa |
- Atmospheric pressure is variable but standardized at 101.325 kPa (760 Torr)
- Low vacuum, also called rough vacuum or coarse vacuum, is vacuum that can be achieved and measured with rudimentary equipment such as a vacuum cleaner and a liquid column manometer.
- Medium vacuum is vacuum that can be achieved with a single pump, but is too low to measure with a liquid or mechanical manometer. It can be measured with a McLeod gauge, thermal gauge or a capacitive gauge.
- High vacuum is vacuum where the MFP of residual gases is longer than the size of the chamber or of the object under test. High vacuum usually requires multi-stage pumping and ion gauge measurement. Some texts differentiate between high vacuum and very high vacuum.
- Ultra high vacuum requires baking the chamber to remove trace gases, and other special procedures.
- Deep space is generally much more empty than any artificial vacuum that we can create.
- Perfect vacuum is an ideal state that cannot be obtained in a laboratory, nor even in outer space.
Examples[]
| Vacuum cleaner | approximately 80 kPa | (600 Torr) |
| liquid ringvacuum pump | approximately 3.2 kPa | (24 Torr) |
| freeze drying | 100 to 10 Pa | (1 to 0.1 Torr) |
| rotary vane pump | 100 Pa to 100 µPa | (1 Torr to 10−6 Torr) |
| Incandescent light bulb | 10 to 1 Pa | (0.1 to 0.01 Torr) |
| Thermos bottle | 1 to 0.1 Pa | (10−2 to 10−3 Torr) |
| Near earth outer space | approximately 100 µPa | (10−6 Torr) |
| CryopumpedMBE chamber | 100 nPa to 1 nPa | (10−9 Torr to 10−11 Torr) |
| Pressure on the Moon | approximately 1 nPa | (10−11 Torr) |
| Interstellar space | approximately 1 fPa | (10−17 Torr) |
Measurement[]
Vacuum is measured in units of pressure. The SI unit of pressure is the Pascal (abbreviation Pa), but vacuum is usually measured in Torrs. A Torr is equal to the displacement of a millimeter of mercury (mmHg) in a manometer, with 1 Torr equaling 133.3223684 Pascal above absolute zero pressure. Vacuum is often also measured using the barometric scale, or as a percentage of atmospheric pressure in bars or atms. Low vacuum is often measured in inches of mercury (inHg) below atmospheric. "Below atmospheric" means that the absolute pressure is equal to the atmospheric pressure (29.92inHg) minus the vacuum pressure in inHg. Thus a vacuum of 26 inHg is equivalent to an absolute pressure of (29.92 - 26) or 3.92 inHg.
Many devices are used to measure the pressure in a vacuum, depending on what range of vacuum is needed.
- Hydrostatic gauges (such as the oil or mercury manometer) consist of various liquids in some sort of tubing to measure pressure. The simplest of these is a closed-end u-shaped tube, one side of which is connected to the region of interest. The height of the liquid in the tube will rise or fall depending on the pressure in the region. Simple hydrostatic gauges are effective in the region of 0.1 torr (see McLeod gauge); more sophisticated versions can measure to about 10−4 Torr.
- Mercury manometer is used in the case of condensers used with steam turbines in Thermal power stations.
- Mechanical gauges depend on a diaphram, usually made of metal, which will change shape in response to the pressure of the region in question. A variation on this idea is the capacitance manometer, in which the diaphram makes up a part of a capacitor. A change in pressure leads to the flexure of the diaphram, which results in a change in capacitance. These gauges are effective from 10−3 Torr to 10−4 Torr.
- Thermal Conductivity gauges rely on the fact that the ability of a gas to conduct heat decreases with pressure. In this type of gauge, a wire filament is heated by running current through it. A thermocouple can then be used to measure the temperature of the filament. This temperature is dependent on the rate at which the filament loses heat to the surrounding gas, and therefore on the thermal conductivity. Another method involves measuring the resistivity of the filament, which is dependent on its temperature. These gauges are accurate from 10 Torr to 10−3 Torr.
- Ion gauges are used in ultrahigh vacua. They come in two types: hot cathode and cold cathode. In the hot cathode version an electrically heated filament produces an electron beam. The electrons travel through the gauge and ionize gas molecules around them. The resulting ions are collected at a negative electrode. The current depends on the number of ions, which depends on the pressure in the gauge. Hot cathode gauges are accurate from 10−3 Torr to 10−10 Torr. The principle behind cold cathode version is the same, except that electrons are produced in a discharge created by a high voltage electrical discharge. Cold Cathode gauges are accurate from 10−2 Torr to 10−9 Torr.
Uses[]

Light bulbs contain a partial vacuum, usually backfilled with argon, which protects the tungsten filament
Vacuum is useful in a variety of processes and devices. Its first common use was in Incandescent light bulbs to protect the tungsten filament from chemical degradation. Its chemical inertness is also useful for vacuum welding, for chemical vapor deposition and Dry Etching in semiconductor fabrication and optical coating fabrication, and for ultra-clean inert storage. The reduction of convection improves the thermal insulation of thermos bottles and double-paned windows. Deep vacuum promotes outgassing which is used in freeze drying, adhesive preparation, steel manufacture, and process purging. The electrical properties of vacuum make electron microscopes and vacuum tubes possible, including cathode ray tubes.
In the 19th century, vacuum was used for traction on Isambard Kingdom Brunel's [3] experimental atmospheric railway [4].
Vacuum Pumping[]
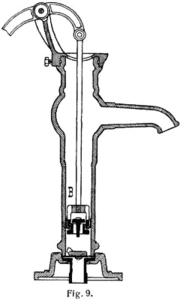
The manual water pump draws water up from a well by creating a vacuum that water rushes in to fill. In a sense, it acts to evacuate the well, although the high leakage rate of dirt prevents a high quality vacuum from being maintained for any length of time.
- Main article: vacuum pump
The easiest way to create an artificial vacuum is to expand the volume of a container. For example, your muscles expand your lungs to create a partial vacuum inside them, and air rushes in to fill the vacuum. By repeatedly closing off a compartment of the vacuum and exhausting it, it is possible to pump air out of a chamber of fixed size. This is the principle behind positive displacement vacuum pumps. Inside the pump, a mechanism expands a small sealed cavity to create a deep vacuum. Because of the pressure differential, some air from the chamber is pushed into the pump's small cavity. The pump's cavity is then sealed from the chamber, opened to the atmosphere, and squeezed back to a minute size. This is how a simple manual water pump works.
Many variations of the positive displacement pump have been developed, and other principles have been used as well. Momentum transfer pumps, which bear some similarities to dynamic pumps used at higher pressures, can achieve much higher quality vacuums than positive displacement pumps. Entrapment pumps can capture gases in a solid or absorbed state, often with no moving parts, no seals and no vibration. None of these pumps are universal; each type has important performance limitations. They all share a difficulty in pumping low molecular weight gases, especially hydrogen, helium, and neon.
The ultimate vacuum that can be attained in a system is also dependent on many things other than the nature of the pumps. Multiple pumps may be connected in series, called stages, to achieve higher vacuums. The choice of seals, chamber geometry, materials, and pump-down procedures will all have an impact. Collectively, these are called vacuum technique. And sometimes, the quality of vacuum is not the only relevant characteristic. Pumping systems differ in oil contamination, vibration, preferential pumping of certain gases, pump-down speeds, intermittent duty cycle, reliability, or tolerance to high leakage rates.
In ultra high vacuum systems, some very odd leakage paths and outgassing sources must be considered. The water absorption of aluminium and palladium becomes an unacceptable source of outgassing, and even the absorptivity of hard metals such as stainless steel or titanium must be considered. Some oils and greases will boil off in extreme vacuums. The porosity of the metallic chamber walls may have to be considered, and the grain direction of the metallic flanges should be parallel to the flange face.
The lowest pressures currently achievable in laboratory are about 10−13 Torr.
Vacuum in space[]
Much of outer space has the density and pressure of an almost perfect vacuum. It has no friction. The properties of the vacuum remain largely unknown.
A perfect vacuum is an ideal state that cannot practically be obtained in a laboratory, nor even in outer space, where there are a few hydrogen atoms per cubic centimeter at 10−14 Pascal or 10−16 Torr.