Fractional distillation is the separation of a mixture of miscible compounds by their boiling point, by heating to high enough temperatures.
Industrial uses of Fractional Distillation[]
- Main article: Oil refinery#operation
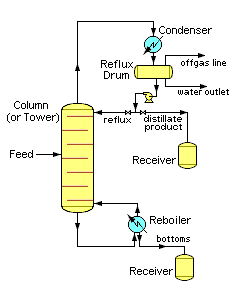
Chemical engineering schematic of industrial Continuous Binary Fractional Distillation
Distillation is the most common form of separation technology in the chemical industry. In most chemical processes, the distillation is continuous steady state, where batch fractionation is not as economical. New feed is always being added to the distillation column and products are always being removed. Unless the process is disturbed due to changes in feed, heat, ambient temperature, or condensing, the amount of feed being added and the amount of product being removed are normally equal. This is known as continuous, steady-state fractional distillation.
Application of continuous distillation[]
- Refineries
The most widely used industrial applications of continuous, steady-state fractional distillation are in petroleum refineries, petrochemical plants and natural gas processing plants.
Typical distillation towers in oil refineries
- Equipment
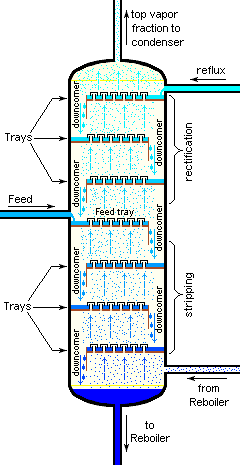
Cross-sectional diagram of operating industrial Distllation Tower with trays
Industrial distillation is typically performed in large, vertical cylindrical columns known as "distillation towers" or "distillation columns" with diameters ranging from about 65 centimeters to 6 meters and heights ranging from about 6 meters to 60 meters or more. The distillation towers have liquid outlets at intervals up the column which allow for the withdrawal of different fractions or products having different boiling points or boiling ranges. The "lightest" products (those with the lowest boiling point) exit from the top of the columns and the "heaviest" products (those with the highest boiling point) exit from the bottom of the column. Large-scale industrial towers also use reflux to achieve more complete separation of products.
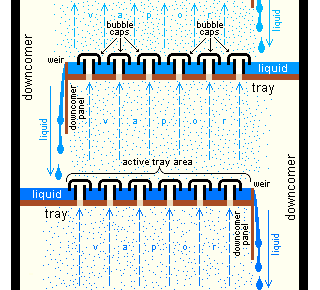
Cross-sectional diagram of a section of above distillation tower showing a couple of Trays with Bubble caps
- Atmospheric air separation
Fractional distillation is also used in air separation, producing liquid oxygen, liquid nitrogen, and high purity argon.
- Others
Distillation of chlorosilanes [1] also enable the production of high-purity silicon [2] for use as a semiconductor.
Column construction details[]
In industrial uses, sometimes a packing material is used in the column instead of trays, especially when low pressure drops across the column are required, as when operating under vacuum. This packing material can either be random dumped packing (1-3" wide) or structured sheet metal. Typical manufacturers are Koch, Sulzer and other companies. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where mass transfer takes place. Unlike conventional tray distillation in which every tray represents a separate point of vapor liquid equilibrium, the vapor liquid equilibrium curve in a packed column is continuous. However, when modeling packed columns it is useful to compute a number of "theoretical stages" to denote the separation efficiency of the packed column with respect to more traditional trays. Differently shaped packings have different surface areas and void space between packings. Both of these factors affect packing performance.
See also[]
References[]
- ^ Kister, Henry Z. (1992). Distillation Design, 1st Edition, McGraw-Hill. 0070349096.
- ^ Perry, Robert H. and Green, Don W. (1984). Perry's Chemical Engineers' Handbook, 6th Edition, McGraw-Hill. 0070494797.
| This page uses Creative Commons Licensed content from Wikipedia (view authors). | 
|