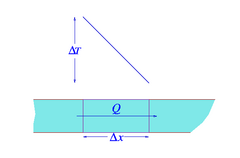
Heat flow along perfectly insulated wire
Heat conduction is the transmission of heat across matter.
Heat transfer is always directed from a higher to a lower temperature. Denser [1] substances are usually better conductors; metals are excellent conductors.
The law of heat conduction also know as Fourier's law states that the time rate of heat flow Q through a slab (or a portion of a perfectly insulated wire, as shown in the figure) is proportional [2] to the gradient [3] of temperature difference:
A is the transversal surface area, is the thickness of the body of matter through which the heat is passing, k is a conductivity constant dependent on the nature of the material and its temperature, and is the temperature difference through which the heat is being transferred. This law forms the basis for the derivation of the heat equation [4]. R-value [5] is the unit for heat resistance, the reciprocal of the conductance. Ohm's law is the electrical analogue of Fourier's law.
Conductance[]
Writing
Fourier's law can also be stated as:
where U is the conductance. The reciprocal of conductance is resistance, equal to:
and it is resistance which is additive when several conducting layers lie between the hot and cool regions, because A and Q are the same for all layers. In a multilayer partition, the total conductance is related to the conductance of its layers by:
So, when dealing with a multilayer partition, the following formula is usually used:
When heat is being conducted from one fluid to another through a barrier, it is sometimes important to consider the conductance of the thin film of fluid which remains stationary next to the barrier. This thin film of fluid is difficult to quantify, its characteristics depending upon complex conditions of turbulence and viscosity, but when dealing with thin high-conductance barriers it can sometimes be quite significant.
Newton's law of cooling[]
A related principle, Newton's law of cooling, states that the rate of heat loss of a body is proportional to the difference in temperatures between the body and its surroundings. This form of heat loss principle, however, is not very precise; a more accurate formulation requires an analysis of heat flow based on the heat equation in an inhomogeneous medium. The general applicability of this simplification is characterized by the Biot number [6].
Nevertheless, it is easy to derive from this principle the exponential decay [7] of temperature of a body. If T is the temperature of the body, then
where r is some positive constant. From which, it follows that
For example, simplified climate models [8] may use Newtonian cooling instead of a full (and computationally expensive) radiation code to maintain atmospheric temperatures.
See also[]
- Heat
- Mpemba effect [9]
- Scientific laws named after people
- Thermal conductivity [10]
- Convection [11]
- Thermal radiation [12]
- Heatpipe
| This page uses Creative Commons Licensed content from Wikipedia (view authors). | 
|









